taylormadeknives
Well-Known Member
I was wondering if anyone has seen this before or if anyone can explain?
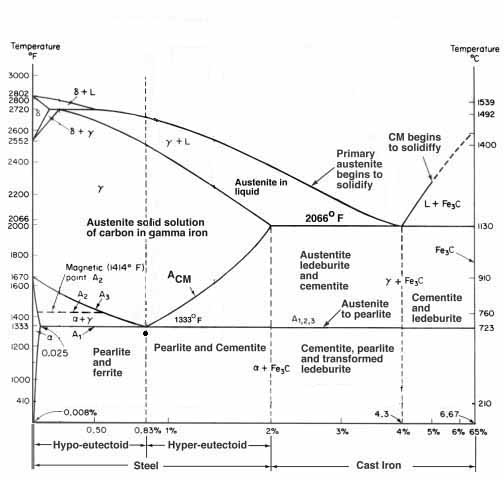
I have done a clay quench on several knives using 1084, 1095. I am no expert by no means. I understand the concept of the hamon. With that being said I don't have a clue what happened here. I have heard that 1084 is particular about a clay quench. I have never had any trouble with it. I figured the 1080 would be same. Wrong. It looks great from a distance, but the surface has a grainy textured look. I haven't seen this before. I heard the term thermal banding, but don't know if this is the case. I should mention this was a stock removal project. I normalized 3 times using my heat treat oven at 1600 F. Then I applied clay and on into oven again the next day. I equalized at 1200 if I remember right, then ramped up to 1475 for austenizing temp. I held at temp for 7 minutes. Not sure about soak time on 1080. Shouldn't be any since the carbon is below .084 correct? So all my carbon should have been into solution at this temp and soak. I then quenched in warm fast quench oil. It is the 11 sec. fast quench oil I got from McMaster Carr. After the quench I tempered at 380 F for 1 hr. Then once again for 1hr. I finished grinding the blade, started my polishing process and ended up with this. Sorry for the lengthy explanation, but I realize there are a lot of variables and I wanted to describe my process. If anyone has seen this before I would like to know. I think it is going to be ok, just not preferred. I may stay away from 1080 for a clay quench if this is a problem. What stumps me is I have had great results with Aldo's 1084. This 1080 came from Tracy here at USA KNIFEMAKER, so there is no doubt it is 1080. I have attached some pics. Any advice, help or tips would be great




Sent from my SPH-L710 using Tapatalk
I have done a clay quench on several knives using 1084, 1095. I am no expert by no means. I understand the concept of the hamon. With that being said I don't have a clue what happened here. I have heard that 1084 is particular about a clay quench. I have never had any trouble with it. I figured the 1080 would be same. Wrong. It looks great from a distance, but the surface has a grainy textured look. I haven't seen this before. I heard the term thermal banding, but don't know if this is the case. I should mention this was a stock removal project. I normalized 3 times using my heat treat oven at 1600 F. Then I applied clay and on into oven again the next day. I equalized at 1200 if I remember right, then ramped up to 1475 for austenizing temp. I held at temp for 7 minutes. Not sure about soak time on 1080. Shouldn't be any since the carbon is below .084 correct? So all my carbon should have been into solution at this temp and soak. I then quenched in warm fast quench oil. It is the 11 sec. fast quench oil I got from McMaster Carr. After the quench I tempered at 380 F for 1 hr. Then once again for 1hr. I finished grinding the blade, started my polishing process and ended up with this. Sorry for the lengthy explanation, but I realize there are a lot of variables and I wanted to describe my process. If anyone has seen this before I would like to know. I think it is going to be ok, just not preferred. I may stay away from 1080 for a clay quench if this is a problem. What stumps me is I have had great results with Aldo's 1084. This 1080 came from Tracy here at USA KNIFEMAKER, so there is no doubt it is 1080. I have attached some pics. Any advice, help or tips would be great




Sent from my SPH-L710 using Tapatalk