You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Austempering - how is it done and what is it' purpose ??????
- Thread starter jwstarr
- Start date
Doug Lester
Well-Known Member
Austempering is done primarily to cause austinite to convert, at least in part, to bainite. Bainite is harder and tougher than pearlite but less hard and tougher than martensite. Here we will be talking about lower bainite. With 52100 you could austemper for 100% bainite formation but still get an HRc of approximately 58 by holding at at just above the Mx point, 500 degrees in this case, for an extended period of time in oil or molten salts. One hour would do for the 52100.
For some steels, I'll use 9260 as an example, holding it at just above the Ms point will not give a high enough hardness. For the 9260 that would be an HRc of about 55. To get the steel harder I could quench the steel in oil or molten salts between the Ms and Mf points for several seconds to get a percentage of martensite formation. Then I would tranfer that to a bath just above the Ms point. For the 9260 that could be about 350-375 degrees for approximately 75% conversion to martensite for an HRc of 58. I would hold the blade in the quenchant with the tang out until the color went out of the tang and then transfere the whole knife to a bath at 500-510 degrees for about three hours to convert the remaining austinite to bainite.
There is a third method where you can quench the steel in oil or molten salts at a little below the Ms point to get a percentage of martensite and then hold at that temperature until the remainder of the austinite converts to bainite. For right now I'm sort of stuck doing that with my 52100 because I can't get the roaster I have to hold a temperature above 450 degrees. This gives me about 25% martensite. Holding for 3-4 hours, just to be safe, will convert the rest of the austinite to bainite and temper the martensite. One of my projects is to see if I can over ride the control on a second eletric roaster that I have so that it will hold in the low 500's.
Not all steels work well with these techniques. Shallow hardening steels have to be thin enough and that is determined by the carbon and manganese levels. Other alloys will also cause problems. As stated above, the 9260 can be quenched between Ms and Mf for a 75% martensite content but if tried to do the third technique and hold it at that temperature until the remainder of the austinite converted it wouldn't work. The temperature would be too low to form bainite. If I raised the temperture to about 400 degrees for a 50% martensite level and an HRc of 56-57 then I could hold it to finish conversion to bainite but it would take about 24 hours. If I had some 4360, a nickel/chrome/moly alloy, I could try the first method of holding it just above the Ms point, about 425 in this case, to get 100% bainite with an HRc of about 58. However, for 4360 it would take about 48 hours.
To choose a steel for this process you will need to obtain an ITT diagram, also refered to as TTT diagrams, for the steel that you will want to consider. Critical temperatures, Ms, Mf, conversion start times, conversion finish times, and times to the nose vary from alloy to alloy. I would also do some studying on Steel Metallurgy before try these methods. Steel Metallurgy for the Non-Metallurgist by John Verhoeven is a good one. ITT diagrams can be obtained from ASM International for a reasonable fee if you can't find a book of diagrams in a library. My local library doesn't have them but I'm sure that I could find them at one of the local colleges if they allow access to the public.
Doug
For some steels, I'll use 9260 as an example, holding it at just above the Ms point will not give a high enough hardness. For the 9260 that would be an HRc of about 55. To get the steel harder I could quench the steel in oil or molten salts between the Ms and Mf points for several seconds to get a percentage of martensite formation. Then I would tranfer that to a bath just above the Ms point. For the 9260 that could be about 350-375 degrees for approximately 75% conversion to martensite for an HRc of 58. I would hold the blade in the quenchant with the tang out until the color went out of the tang and then transfere the whole knife to a bath at 500-510 degrees for about three hours to convert the remaining austinite to bainite.
There is a third method where you can quench the steel in oil or molten salts at a little below the Ms point to get a percentage of martensite and then hold at that temperature until the remainder of the austinite converts to bainite. For right now I'm sort of stuck doing that with my 52100 because I can't get the roaster I have to hold a temperature above 450 degrees. This gives me about 25% martensite. Holding for 3-4 hours, just to be safe, will convert the rest of the austinite to bainite and temper the martensite. One of my projects is to see if I can over ride the control on a second eletric roaster that I have so that it will hold in the low 500's.
Not all steels work well with these techniques. Shallow hardening steels have to be thin enough and that is determined by the carbon and manganese levels. Other alloys will also cause problems. As stated above, the 9260 can be quenched between Ms and Mf for a 75% martensite content but if tried to do the third technique and hold it at that temperature until the remainder of the austinite converted it wouldn't work. The temperature would be too low to form bainite. If I raised the temperture to about 400 degrees for a 50% martensite level and an HRc of 56-57 then I could hold it to finish conversion to bainite but it would take about 24 hours. If I had some 4360, a nickel/chrome/moly alloy, I could try the first method of holding it just above the Ms point, about 425 in this case, to get 100% bainite with an HRc of about 58. However, for 4360 it would take about 48 hours.
To choose a steel for this process you will need to obtain an ITT diagram, also refered to as TTT diagrams, for the steel that you will want to consider. Critical temperatures, Ms, Mf, conversion start times, conversion finish times, and times to the nose vary from alloy to alloy. I would also do some studying on Steel Metallurgy before try these methods. Steel Metallurgy for the Non-Metallurgist by John Verhoeven is a good one. ITT diagrams can be obtained from ASM International for a reasonable fee if you can't find a book of diagrams in a library. My local library doesn't have them but I'm sure that I could find them at one of the local colleges if they allow access to the public.
Doug
Doug Lester
Well-Known Member
I've got three text books on metallurgy and it still can make my head spin. Some of it is just getting the terminology down. I used to think that the ITT diagrams were unfathomable but they are really pretty easy once you see a diagram with explanations. I still can't get my head around CCT, continuous cooling transfomation, diagrams.
Doug
Doug
McClellan Made Blades
Well-Known Member
I've got three text books on metallurgy and it still can make my head spin. Some of it is just getting the terminology down. I used to think that the ITT diagrams were unfathomable but they are really pretty easy once you see a diagram with explanations. I still can't get my head around CCT, continuous cooling transfomation, diagrams.
Doug
Doug would happen to have a TTT diagram with the definitions? I have looked at those several times, and came away with more questions than answers! It sure be appreciated, and I'm sure there are some other folks that might like ot see it, Thanks Bud, Rex
Doug Lester
Well-Known Member
Arg!! Let me see if I can work something up with my graphics program. I don't want to violate anyone's copywright by scanning a page from any of those books that I have. Give me a couple of days and I think that I should b e able to work up something generic.
One thing that helped me finally understand what I was looking at with the TTT/ITT/IT diagrams is to realize how they were generated. The researchers took a 1/4" rod of a specific alloy to be tested and cut a bunch of 1/32" thick wafers and austinized them at the same temperature and then quenched them all to a specific temperature below the upper critical temperature and above the Mf temperature. Then at set intervals they quench in water and examine the wafer for conversion products of austinite, i.e. ferrite, pearlite, bainite, and martensite to infer what crystaline forms of steel existed at a given time at that given temperature. They also do that at other temperatures between the upper critical temperature and the Mf point and plot the points on the graf for the appearance of different conversion products. Just setting here typing I think I've come up with a way to do it. I just need a few days to play with my graphics program.
Doug
One thing that helped me finally understand what I was looking at with the TTT/ITT/IT diagrams is to realize how they were generated. The researchers took a 1/4" rod of a specific alloy to be tested and cut a bunch of 1/32" thick wafers and austinized them at the same temperature and then quenched them all to a specific temperature below the upper critical temperature and above the Mf temperature. Then at set intervals they quench in water and examine the wafer for conversion products of austinite, i.e. ferrite, pearlite, bainite, and martensite to infer what crystaline forms of steel existed at a given time at that given temperature. They also do that at other temperatures between the upper critical temperature and the Mf point and plot the points on the graf for the appearance of different conversion products. Just setting here typing I think I've come up with a way to do it. I just need a few days to play with my graphics program.
Doug
Doug Lester
Well-Known Member
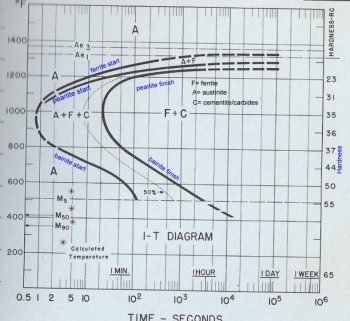
Ok, this is the best that I can do, my graphics program won't allow me to draw my own pictures so I skanned an IT diagram to use as a generic sample and labled it up. The one I chose is for a hypoeutectic steel, less than 77 points of carbon, but I will explain how it differs from a eutictic, 77 points of carbon, or a hypereutectic, more than 77 points of carbon, steel.
The diagram is a graf of time and temperature with the time in log scale. There is a scale of hardness on the right side of the diagram that gives the hardness, usually in HRc, that indicated the hardness that will be achieved if the austinite is allowed to convert completely at the temperature opposite it on the left side of the diagram. The apex in the curve of the line on the left is refred to as the nose. That thing that you have to beat with the actual curve in the steel when you quench it. You will also note that between the start and finish lines there is a 50% line. That's just the half way point for the conversion of austinite at a given temperature. Where the lines are broken rather than solid it means that the data was calculated or the data points generated were not clear.
Remember that this is a chart of how the austinite converts to other products and how long it takes it to convert at a given temperature. So for the steel diagramed at 1100 degrees the steel will start to convert to ferrite in about two seconds. In about 3-4 seconds it will start to convert to pearlite. In 11-12 seconds at that temperature the austinite is completely converted ferrite and carbides, which incluses cemementite. So you may ask yourself where did the pearitle go. Remember, that a combination of ferrite and cementite in altenating plates; it's there. For hypereutectic steels the C will also include cementite that is outside of the pearlite as well as other carbides.
The reason that ferrite starts to form before the pearlite in hypoeutectic steels is because the austinite has to give up carbon when it converts to ferrite and that carbon will go back into solution until the remaining austinite is saturated. Until the austinite is saturated with carbon pearlite cannot start to form. This line will not appear in eutictic or hypereutictic steels because the austinite crystals start out satureated. Sometimes you will also find an IT diagram for a hypoeutictic steel that does not show a ferrite start line. That just a quirk of the person preparing the diagram. That reagon is still there. Also, for steels at or above the eutectic point the Ae3 line, upper critical, is not listed.
Now you will notice that the lines above the nose of the curve are the pearlite start and pearlite finish lines and the lines below the nose of the curve are the bainite start and bainite finish lines. That is because pearlite, in general, forms above the nose of the curve and bainite below it. Also upper bainite forms closer to the nose and lower bainite forms closer to the martensite start line (Ms). These areas overlap so there are regions where pearlite and upper bainite form at the same time and regions where upper and lower bainite form at the same time.
The Ms point is the temperature where martensite starts to form. The M90 point on the diagram is the temperature when 90% of the austinite converts to martensite. This is more or less the martensite finish point for most common steel. For some of the more complex steels such as the stainless steels the actual martensite finish point my be well below freezing. For the more simple tool and spring steel that point is above room temperature.
The IT diagrams are used by immagining the actual cooling curve of the steel layed over it and trying to estimate what will happen to the crystaline structere of the steel in our quenching methods. As you see with this steel you only have about 0.8 seconds to miss the tip of the nose at about 1000 degrees to prevent formation of pearlite and/or bainite. For more complex steels the nose of the curve will be farther to the right. Take 52100 for example. It has2-3 seconds at about the same temperature to miss the nose of the curve. I have a diagram for a low carbon stainless steel that has around three minutes to miss the nose of the curve. Steels like that would be air quenching.
The nose of the curve with something like L6 may have too much time to allow all the austinite to convert to pearlite or bainite when it is air cooled and the actual cooling line will cross back outside the curve allowing the remainder of the austinite to cross over the Ms line and form a low amount of martinsite. That's the annoying "partially air quenching" factor.
The shapes of these lines can vary a good deal with the different alloys. Also the temperatures for the critical points, such as the A1, A3, Ms, and Mf points will vary according to the characteristics of the alloy as well as where to nose of the curve is in relation to time and temperature.
I hope I haven't made my explanation look like a soup sandwich. I'll try to answere any questions.
Doug:steve:
